Hydrogen bonding in water
Hydrogen bonds in water
Introduction to the properties of water
You are a tool-making, communicating, learning bag of water. Okay, that’s not completely fair, but it's close since the human body is 60 to 70% water. And it's not just humans—most animals and even tiny bacteria are made up mostly of water\[^1\]. Water is key to the existence of life as we know it. That may sound dramatic, but it’s true—and dramatic things that are true are what make life interesting! Most of an organism’s cellular chemistry and metabolism occur in the water-based “goo” inside its cells, called cytosol.
Water is not only very common in the bodies of organisms, but it also has some unusual chemical properties that make it very good at supporting life. These properties are important to biology on many different levels, from cells to organisms to ecosystems. You can learn more about the life-sustaining properties of water in the following articles:
Solvent properties of water: Learn how and why water dissolves many polar and charged molecules.
Cohesion and adhesion of water: Water can stick to itself (cohesion) and other molecules (adhesion).
Specific heat, heat of vaporization, and density of water: Water has a high heat capacity and heat of vaporization, and ice—solid water—is less dense than liquid water.
Water owes these unique properties to the polarity of its molecules and, specifically, to their ability to form hydrogen bonds with each other and with other molecules. Below, we'll look at how this hydrogen bonding works.
Polarity of water molecules
The key to understanding water’s chemical behavior is its molecular structure. A water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. All of the electron pairs—shared and unshared—repel each other.
The most stable arrangement is the one that puts them farthest apart from each other: a tetrahedron, with the \[\text{O}-\text{H}\] bonds forming two out of the four “legs”. The lone pairs are slightly more repulsive than the bond electrons, so the angle between the \[\text{O}-\text{H}\] bonds is slightly less than the 109° of a perfect tetrahedron, around 104.5°.\[^2\]
Because oxygen is more electronegative—electron-greedy—than hydrogen, the \[\text{O}\] atom hogs electrons and keeps them away from the \[\text{H}\] atoms. This gives the oxygen end of the water molecule a partial negative charge, while the hydrogen end has a partial positive charge. Water is classified as a polar molecule because of its polar covalent bonds and its bent shape\[^{2,3}\].
Hydrogen bonding of water molecules
Thanks to their polarity, water molecules happily attract each other. The plus end of one—a hydrogen atom—associates with the minus end of another—an oxygen atom.
These attractions are an example of hydrogen bonds, weak interactions that form between a hydrogen with a partial positive charge and a more electronegative atom, such as oxygen. The hydrogen atoms involved in hydrogen bonding must be attached to electronegative atoms, such as \[\text{O}\], \[\text{N}\], or \[\text{F}\].

Water molecules are also attracted to other polar molecules and to ions. A charged or polar substance that interacts with and dissolves in water is said to be hydrophilic: hydro means "water," and philic means "loving." In contrast, nonpolar molecules like oils and fats do not interact well with water. They separate from it rather than dissolve in it and are called hydrophobic: phobic means "fearing." You may have noticed this as a not-so-handy feature of oil and vinegar salad dressings. Vinegar is basically just water with a bit of acid.
Water as a solvent
Solvent properties of water
Introduction
Has life ever given you lemons? If so, you've no doubt followed the old adage and made lemonade - involving, of course, a lot of sugar! If you've stirred sugar into lemonade (or tea, or any other water-based drink) and watched it dissolve, then you've already seen the solvent properties of water in action. A solvent is simply a substance that can dissolve other molecules and compounds, which are known as solutes. A homogeneous mixture of solvent and solute is called a solution, and much of life’s chemistry takes place in aqueous solutions, or solutions with water as the solvent.
Because of its polarity and ability to form hydrogen bonds, water makes an excellent solvent, meaning that it can dissolve many different kinds of molecules. Most of the chemical reactions important to life take place in a watery environment inside of cells, and water's capacity to dissolve a wide variety of molecules is key in allowing these chemical reactions to take place.
Solvent properties of water
Thanks to its ability to dissolve a wide range of solutes, water is sometimes called the "universal solvent." However, this name isn't entirely accurate, since there are some substances (such as oils) that don't dissolve well in water. Generally speaking, water is good at dissolving ions and polar molecules, but poor at dissolving nonpolar molecules. (A polar molecule is one that's neutral, or uncharged, but has an asymmetric internal distribution of charge, leading to partially positive and partially negative regions.)
Water interacts differently with charged and polar substances than with nonpolar substances because of the polarity of its own molecules. Water molecules are polar, with partial positive charges on the hydrogens, a partial negative charge on the oxygen, and a bent overall structure. The unequal charge distribution in a water molecule reflects the greater electronegativity, or electron-greediness, of oxygen relative to hydrogen: the shared electrons of the O-H bonds spend more time with the O atom than with the Hs. In the image below, the partial positive and partial negative charges on a water molecule are represented by the symbols δ\[^+\] and δ\[^-\], respectively.
Because of its polarity, water can form electrostatic interactions (charge-based attractions) with other polar molecules and ions. The polar molecules and ions interact with the partially positive and partially negative ends of water, with positive charges attracting negative charges (just like the + and - ends of magnets). When there are many water molecules relative to solute molecules, as in an aqueous solution, these interactions lead to the formation of a three-dimensional sphere of water molecules, or hydration shell, around the solute. Hydration shells allow particles to be dispersed (spread out) evenly in water.
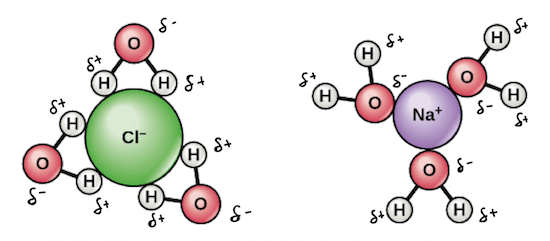
Image modified from "Water: Figure 3," by OpenStax College, Biology (CC BY 3.0).
How does the formation of a hydration shell cause a solute to dissolve? As an example, let's consider what happens to an ionic compound, such as table salt (NaCl), when it's added to water.
If you stir table salt into water, the crystal lattice of NaCl will begin to dissociate into Na\[^+\] and Cl\[^-\] ions. (Dissociation is just a name for the process in which a compound or molecule breaks apart to form ions.) Water molecules form hydration shells around the ions: positively charged Na\[^+\] ions are surrounded by partial negative charges from the oxygen ends of the water molecules, while negatively charged Cl\[^-\] ions are surrounded by partial positive charges from the hydrogen ends. As the process continues, all of the ions in the table salt crystals are surrounded by hydration shells and dispersed in solution.
Nonpolar molecules, like fats and oils, don't interact with water or form hydration shells. These molecules don't have regions of partial positive or partial negative charge, so they aren't electrostatically attracted to water molecules. Thus, rather than dissolving, nonpolar substances (such as oils) stay separate and form layers or droplets when added to water.
