The history of atomic chemistry
Dalton's atomic theory
Key Points
Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.
Dalton based his theory on the law of conservation of mass and the law of constant composition.
The first part of his theory states that all matter is made of atoms, which are indivisible.
The second part of the theory says all atoms of a given element are identical in mass and properties.
The third part says compounds are combinations of two or more different types of atoms.
The fourth part of the theory states that a chemical reaction is a rearrangement of atoms.
Parts of the theory had to be modified based on the discovery of subatomic particles and isotopes.
Chemists ask questions.
Chemistry is full of unanswered questions. One of the first questions people have been asking since ancient times is What is the world made of?
That is, if we were to zoom in ~100000000000 times—that is 11 zeros!—on the skin of your fingertip, what would we see? Would that look any different from zooming in on, say, an apple? If we then cut up the apple into tinier and tinier pieces using an imaginary tiny knife, would we reach a point where the pieces could no longer be cut any smaller? What would those pieces look like, and would they still have apple properties?
The answers to these questions are fundamental to modern chemistry, and chemists didn't agree on the answer until a few hundred years ago. Thanks to scientists such as John Dalton, modern chemists think of the world in terms of atoms. Even if we can't see atoms with our naked eye, properties of matter such as color, phase (e.g., solid, liquid, gas), and even smell come from interactions on an atomic level. This article will discuss John Dalton's atomic theory, which was the first complete attempt to describe all matter in terms of atoms and their properties.
Basis for Dalton's theory
Dalton based his theory on two laws: the law of conservation of mass and the law of constant composition.
The law of conservation of mass says that matter is not created or destroyed in a closed system. That means if we have a chemical reaction, the amount of each element must be the same in the starting materials and the products. We use the law of conservation of mass every time we balance equations!
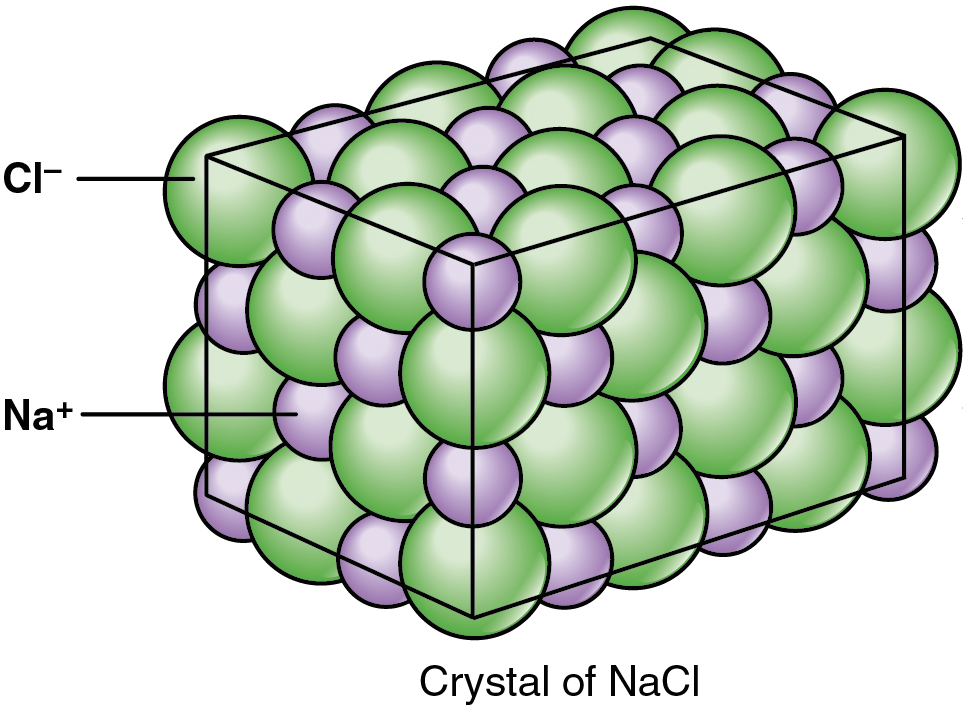
A chemist thinks of table salt as sodium and chloride ions arranged in a crystal lattice structure. Image credit: "Image of salt" by OpenStax Anatomy and Physiology, CC-BY-NC-SA 4.0.
The law of constant composition says that a pure compound will always have the same proportion of the same elements. For example, table salt, which has the molecular formula \[\text{NaCl}\], contains the same proportions of the elements sodium and chlorine no matter how much salt you have or where the salt came from. If we were to combine some sodium metal and chlorine gas—which I wouldn't recommend doing at home—we could make more table salt which will have the same composition.
Concept check: A time-travelling scientist from the early 1700s decides to run the following experiment: he takes a 10 gram sample of ethanol (\[\text{CH}_3 \text{CH}_2 \text{OH}\]) and burns it in the presence of oxygen in an open beaker. After the reaction is done, the beaker is empty. Does this result violate the law of conservation of mass?
Dalton's atomic theory
Part 1: All matter is made of atoms.
Dalton hypothesized that the law of conservation of mass and the law of definite proportions could be explained using the idea of atoms. He proposed that all matter is made of tiny indivisible particles called atoms, which he imagined as "solid, massy, hard, impenetrable, movable particle(s)".
It is important to note that since Dalton did not have the necessary instruments to see or otherwise experiment on individual atoms, he did not have any insight into whether they might have any internal structure. We might visualize Dalton's atom as a piece in a molecular modeling kit, where different elements are spheres of different sizes and colors. While this is a handy model for some applications, we now know that atoms are far from being solid spheres.
Part 2: All atoms of a given element are identical in mass and properties.
Dalton proposed that every single atom of an element, such as gold, is the same as every other atom of that element. He also noted that the atoms of one element differ from the atoms of all other elements. Today, we still know this to be mostly true. A sodium atom is different from a carbon atom. Elements may share some similar boiling points, melting points, and electronegativities, but no two elements have the same exact set of properties.
Why is this only MOSTLY true?

A basic molecular modeling kit, including spherical atoms of different size and color that can be connected by sticks to represent chemical bonds. Image credit: "Photo of modeling kit" by Sonia on Wikimedia Commons, CC-BY 3.0
Part 3: Compounds are combinations of two or more different types of atoms.
In the third part of Dalton's atomic theory, he proposed that compounds are combinations of two or more different types of atoms. An example of such a compound is table salt. Table salt is a combination of two separate elements with unique physical and chemical properties. The first, sodium, is a highly reactive metal. The second, chlorine, is a toxic gas. When they react, the atoms combine in a 1:1 ratio to form white crystals of \[\text{NaCl}\], which we can sprinkle on our food.
Since atoms are indivisible, they will always combine in simple whole number ratios. Therefore, it would not make sense to write a formula such as \[\text{Na}_{0.5}\text{Cl}_{0.5}\] because you can't have half of an atom!
Part 4: A chemical reaction is a rearrangement of atoms.
In the fourth and final part of Dalton's atomic theory, he suggested that chemical reactions don't destroy or create atoms. They merely rearranged the atoms. Using our salt example again, when sodium combines with chlorine to make salt, both the sodium and chlorine atoms still exist. They simply rearrange to form a new compound.
What have we learned since Dalton proposed his theory?
The short answer: a lot! For instance, we now know that atoms are not indivisible—as stated in part one—because they are made up of protons, neutrons, and electrons. The modern picture of an atom is very different from Dalton's "solid, massy" particle. In fact, experiments by Ernest Rutherford, Hans Geiger, and Ernest Marsden showed that atoms are mostly made up of empty space.
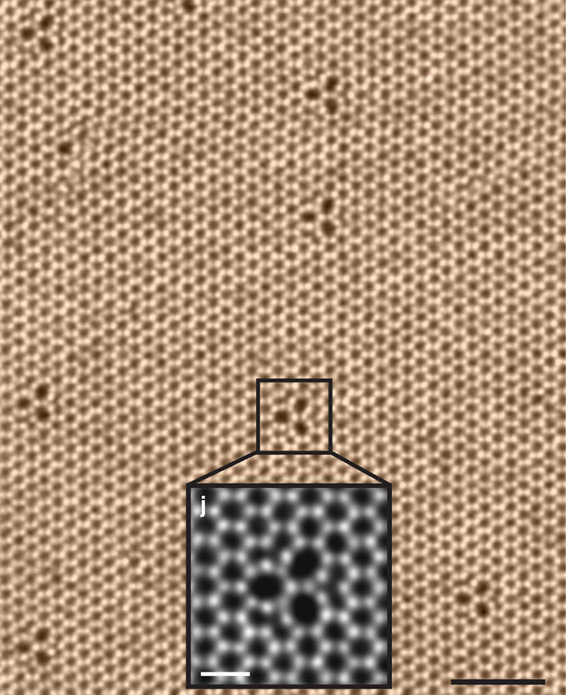
Scanning transmission electron microscopy (STEM) allows us to see the atomic level structure of tungsten selenide, WSe\[_2\]. Image credit: "STEM image" by Kazu Suenaga et al. on Wikimedia Commons, CC BY 4.0
Part two of Dalton's theory had to be modified after mass spectrometry experiments demonstrated that atoms of the same element can have different masses because the number of neutrons can vary for different isotopes of the same element. For more on isotopes, you can watch this video on atomic number, mass number, and isotopes.
Despite these caveats, Dalton's atomic theory is still mostly true, and it forms the framework of modern chemistry. Scientists have even developed the technology to see the world on an atomic level!
Summary
Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.
Dalton based his theory on the law of conservation of mass and the law of constant composition.
The first part of his theory states that all matter is made of atoms, which are indivisible.
The second part of the theory says all atoms of a given element are identical in mass and properties.
The third part says compounds are combinations of two or more different types of atoms.
The fourth part of the theory states that a chemical reaction is a rearrangement of atoms.
Parts of the theory had to be modified based on the existence of subatomic particles and isotopes.
Discovery of the electron and nucleus
Key points
J.J. Thomson's experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons.
Thomson's plum pudding model of the atom had negatively-charged electrons embedded within a positively-charged "soup."
Rutherford's gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus.
Based on these results, Rutherford proposed the nuclear model of the atom.
Introduction: Building on Dalton's atomic theory
In a previous article on Dalton's atomic theory, we discussed the following postulates:
All matter is made of indivisible particles called atoms, which cannot be created or destroyed.
Atoms of the same element have identical mass and physical properties.
Compounds are combinations of atoms of \[2\] or more elements.
All chemical reactions involve the rearrangement of atoms.
Dalton's ideas proved foundational to modern atomic theory. However, one of his underlying assumptions was later shown to be incorrect. Dalton thought that atoms were the smallest units of matter\[-\]tiny, hard spheres that could not be broken down any further. This assumption persisted until experiments in physics showed that the atom was composed of even smaller particles. In this article, we will discuss some of the key experiments that led to the discovery of the electron and the nucleus.
J.J. Thomson and the discovery of the electron
In the late \[19^{\text{th}}\] century, physicist J.J. Thomson began experimenting with cathode ray tubes. Cathode ray tubes are sealed glass tubes from which most of the air has been evacuated. A high voltage is applied across two electrodes at one end of the tube, which causes a beam of particles to flow from the cathode (the negatively-charged electrode) to the anode (the positively-charged electrode). The tubes are called cathode ray tubes because the particle beam or "cathode ray" originates at the cathode. The ray can be detected by painting a material known as phosphors onto the far end of the tube beyond the anode. The phosphors spark, or emit light, when impacted by the cathode ray.
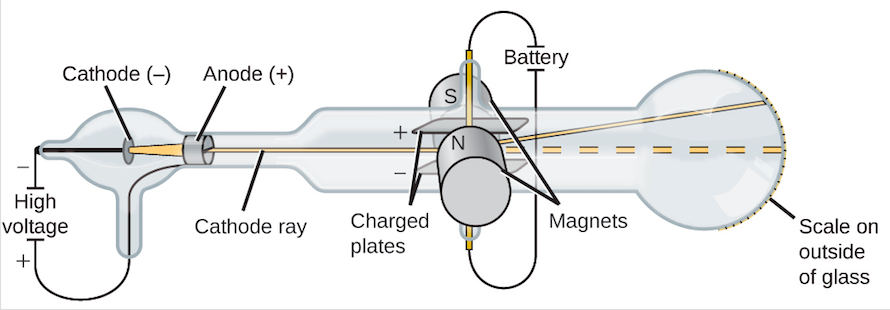
A diagram of J.J. Thomson's cathode ray tube. The ray originates at the cathode and passes through a slit in the anode. The cathode ray is deflected away from the negatively-charged electric plate, and towards the positively-charged electric plate. The amount by which the ray was deflected by a magnetic field helped Thomson determine the mass-to-charge ratio of the particles. Image from Openstax, CC BY 4.0.
To test the properties of the particles, Thomson placed two oppositely-charged electric plates around the cathode ray. The cathode ray was deflected away from the negatively-charged electric plate and towards the positively-charged plate. This indicated that the cathode ray was composed of negatively-charged particles.
Thomson also placed two magnets on either side of the tube, and observed that this magnetic field also deflected the cathode ray. The results of these experiments helped Thomson determine the mass-to-charge ratio of the cathode ray particles, which led to a fascinating discovery\[-\]the mass of each particle was much, much smaller than that of any known atom. Thomson repeated his experiments using different metals as electrode materials, and found that the properties of the cathode ray remained constant no matter what cathode material they originated from. From this evidence, Thomson made the following conclusions:
The cathode ray is composed of negatively-charged particles.
The particles must exist as part of the atom, since the mass of each particle is only \[\sim\]\[\dfrac{1}{2000}\] the mass of a hydrogen atom.
These subatomic particles can be found within atoms of all elements.
While controversial at first, Thomson's discoveries were gradually accepted by scientists. Eventually, his cathode ray particles were given a more familiar name: electrons. The discovery of the electron disproved the part of Dalton's atomic theory that assumed atoms were indivisible. In order to account for the existence of the electrons, an entirely new atomic model was needed.
Concept check: Why did Thomson conclude that electrons could be found in atoms of all elements?
Show the answer
The plum pudding model
Thomson knew that atoms had an overall neutral charge. Therefore, he reasoned that there must be a source of positive charge within the atom to counterbalance the negative charge on the electrons. This led Thomson to propose that atoms could be described as negative particles floating within a soup of diffuse positive charge. This model is often called the plum pudding model of the atom, due to the fact that its description is very similar to plum pudding, a popular English dessert (see image below).
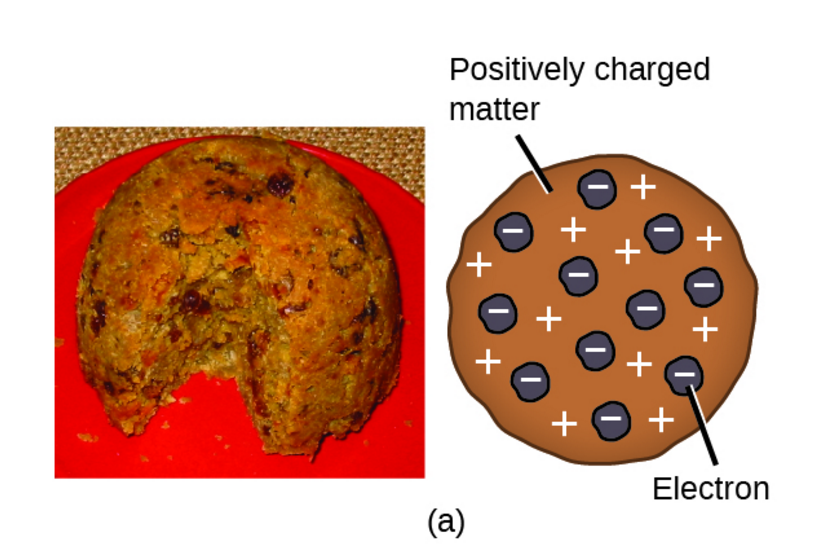
The plum pudding model depicts the electrons as negatively-charged particles embedded in a sea of positive charge. The structure of Thomson's atom is analogous to plum pudding, an English dessert (left). Image from Openstax, CC BY 4.0.
Given what we know now about the actual structure of atoms, this model might sound a little far-fetched. Luckily, scientists continued to investigate the structure of the atom, including testing the validity of Thomson's plum pudding model.
Concept check: Thomson proposed an atomic model with distinct negative charges floating within a "sea" of positive charge. Can you think of another model of the atom that would explain Thomson's experimental results?
Show the answer
Ernest Rutherford and the gold foil experiment
The next groundbreaking experiment in the history of the atom was performed by Ernest Rutherford, a physicist from New Zealand who spent most of his career in England and Canada. In his famous gold foil experiment, Rutherford fired a thin beam of \[\alpha\] particles (pronounced alpha particles) at a very thin sheet of pure gold. Alpha particles are helium nuclei \[(_2^4\text{He}^{2+})\], and they are given off in various radioactive decay processes. In this case, Rutherford placed a sample of radium (a radioactive metal) inside a lead box with a small pinhole in it. Most of the radiation was absorbed by the lead, but a thin beam of \[\alpha\] particles escaped out of the pinhole in the direction of the gold foil. The gold foil was surrounded by a detector screen that would flash when hit with an \[\alpha\] particle.
Why was the foil made out of gold? Couldn't he have saved a buck and used nickel?
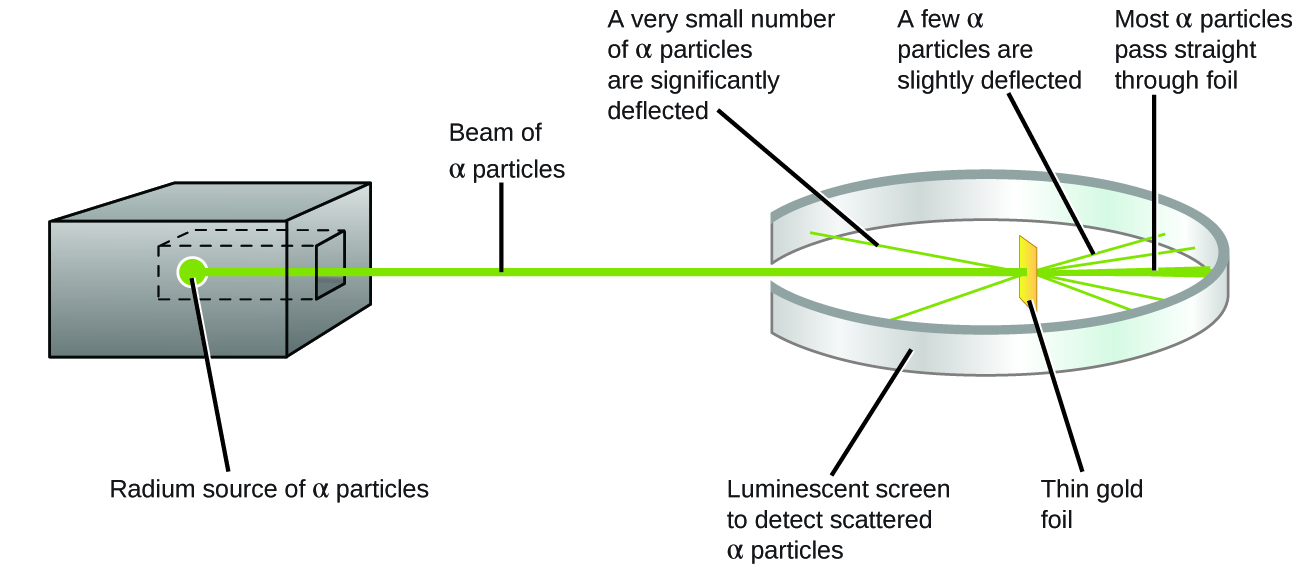
In Rutherford's gold foil experiment, a beam of \[\alpha\] particles that was shot at a thin sheet of gold foil. Most of the \[\alpha\] particles passed straight through the gold foil, but a small number were deflected slightly, and an even smaller fraction were deflected more than \[90^{\circ}\] from their path. Image from Openstax, CC BY 4.0.
Based on Thomson's plum pudding model, Rutherford predicted that most of the \[\alpha\] particles would pass straight through the gold foil. This is because the positive charge in the plum pudding model was assumed to be spread out throughout the entire volume of the atom. Therefore, the electric field from the positively charged "soup" would be too weak to significantly affect the path of the relatively massive and fast-moving \[\alpha\] particles.
The results of the experiment, however, were striking. While almost all of the \[\alpha\] particles passed straight through the gold foil, a few \[\alpha\] particles (about \[1\] in \[20\],\[000\]) were deflected more than \[90^{\circ}\] from their path! Rutherford himself described the results with the following analogy: "It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a \[15\]-\[\text{inch}\] shell at a piece of tissue paper and it came back and hit you."
Based on the plum pudding model of the atom, it was assumed that there was nothing dense or heavy enough inside the gold atoms to deflect the massive \[\alpha\] particles from their paths (see left image). However, what Rutherford actually observed did not match his prediction (see image on right)\[-\]a new atomic model was needed!
The nuclear model of the atom
Based on his experimental results, Rutherford made the following conclusions about the structure of the atom:
The positive charge must be localized over a very tiny volume of the atom, which also contains most of the atom's mass. This explained how a very small fraction of the \[\alpha\] particles were deflected drastically, presumably due to the rare collision with a gold nucleus.
Since most of the \[\alpha\] particles passed straight through the gold foil, the atom must be made up of mostly empty space!
The nuclear model of the atom. Image of Rutherford atom from Wikimedia Commons, CC-BY-SA-3.0.
This led Rutherford to propose the nuclear model, in which an atom consists of a very small, positively charged nucleus surrounded by the negatively charged electrons. Based on the number of \[\alpha\] particles deflected in his experiment, Rutherford calculated that the nucleus took up a tiny fraction of the volume of the atom.
The nuclear model explained Rutherford's experimental results, but it also raised further questions. For example, what were the electrons doing in the atom? How did the electrons keep themselves from collapsing into the nucleus, since opposite charges attract? Luckily, science was ready for the challenge! Physicists such as Niels Bohr continued to design experiments to test the nuclear model of the atom, which eventually evolved into the modern quantum mechanical model.
Summary
J.J. Thomson's experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons.
Thomson proposed the plum pudding model of the atom, which had negatively-charged electrons embedded within a positively-charged "soup."
Rutherford's gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus.
Based on these results, Rutherford proposed the nuclear model of the atom.


