Introduction to kinetics
Activation energy
Introduction
Imagine waking up on a day when you have lots of fun stuff planned. Does it ever happen that, despite the exciting day that lies ahead, you need to muster some extra energy to get yourself out of bed? Once you’re up, you can coast through the rest of the day, but there’s a little hump you have to get over to reach that point.
The activation energy of a chemical reaction is kind of like that “hump” you have to get over to get yourself out of bed. Even energy-releasing (exergonic) reactions require some amount of energy input to get going, before they can proceed with their energy-releasing steps. This initial energy input, which is later paid back as the reaction proceeds, is called the activation energy and is abbreviated \[\text E_{\text A}\].
Activation energy
Why would an energy-releasing reaction with a negative ∆G need energy to proceed? To understand this, we need to look at what actually happens to reactant molecules during a chemical reaction. In order for the reaction to take place, some or all of the chemical bonds in the reactants must be broken so that new bonds, those of the products, can form. To get the bonds into a state that allows them to break, the molecule must be contorted (deformed, or bent) into an unstable state called the transition state. The transition state is a high-energy state, and some amount of energy – the activation energy – must be added in order for the molecule to reach it. Because the transition state is unstable, reactant molecules don’t stay there long, but quickly proceed to the next step of the chemical reaction.
In general, the transition state of a reaction is always at a higher energy level than the reactants or products, such that \[\text E_{\text A}\] always has a positive value – independent of whether the reaction is endergonic or exergonic overall. The activation energy shown in the diagram below is for the forward reaction (reactants \[\rightarrow\] products), which is exergonic. If the reaction were to proceed in the reverse direction (endergonic), the transition state would remain the same, but the activation energy would be larger. This is because the product molecules are lower-energy and would thus need more energy added to reach the transition state at the top of the reaction “hill.” (An activation energy arrow for the reverse reaction would extend from the products up to the transition state.)
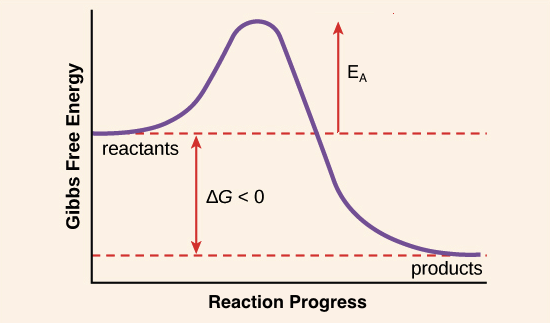
Image modified from OpenStax Biology.
The source of activation energy is typically heat, with reactant molecules absorbing thermal energy from their surroundings. This thermal energy speeds up the motion of the reactant molecules, increasing the frequency and force of their collisions, and also jostles the atoms and bonds within the individual molecules, making it more likely that bonds will break. Once a reactant molecule absorbs enough energy to reach the transition state, it can proceed through the remainder of the reaction.
Activation energy and reaction rate
The activation energy of a chemical reaction is closely related to its rate. Specifically, the higher the activation energy, the slower the chemical reaction will be. This is because molecules can only complete the reaction once they have reached the top of the activation energy barrier. The higher the barrier is, the fewer molecules that will have enough energy to make it over at any given moment.
Many reactions have such high activation energies that they basically don't proceed at all without an input of energy. For instance, the combustion of a fuel like propane releases energy, but the rate of reaction is effectively zero at room temperature. (To be clear, this is a good thing – it wouldn't be so great if propane canisters spontaneously combusted on the shelf!) Once a spark has provided enough energy to get some molecules over the activation energy barrier, those molecules complete the reaction, releasing energy. The released energy helps other fuel molecules get over the energy barrier as well, leading to a chain reaction.
Most chemical reactions that take place in cells are like the hydrocarbon combustion example: the activation energy is too high for the reactions to proceed significantly at ambient temperature. At first, this seems like a problem; after all, you can’t set off a spark inside of a cell without causing damage. Fortunately, it’s possible to lower the activation energy of a reaction, and to thereby increase reaction rate. The process of speeding up a reaction by reducing its activation energy is known as catalysis, and the factor that's added to lower the activation energy is called a catalyst. Biological catalysts are known as enzymes, and we’ll examine them in detail in the next section.
Enzymes
Enzyme reaction velocity and pH
Enzymes and the active site
effects on enzyme activity.
Introduction
As a kid, I wore glasses and desperately wanted a pair of contact lenses. When I was finally allowed to get contacts, part of the deal was that I had to take very, very good care of them, which meant washing them with cleaner every day, storing them in a sterile solution, and, once a week, adding a few drops of something called “enzymatic cleaner.” I didn’t know exactly what “enzymatic cleaner” meant, but I did learn that if you forgot you’d added it and accidentally put your contacts in your eyes without washing them, you were going to have burning eyes for a good fifteen minutes.
As I would later learn, all that “enzymatic” meant was that the cleaner contained one or more enzymes, proteins that catalyzed particular chemical reactions – in this case, reactions that broke down the film of eye goo that accumulated on my contacts after a week of use. (Presumably, the reason it stung when I got it in my eyes was that the enzymes would also happily break down eye goo in an intact eye.) In this article, we’ll look in greater depth at what an enzyme is and how it catalyzes a particular chemical reaction.
Enzymes and activation energy
A substance that speeds up a chemical reaction—without being a reactant—is called a catalyst. The catalysts for biochemical reactions that happen in living organisms are called enzymes. Enzymes are usually proteins, though some ribonucleic acid (RNA) molecules act as enzymes too.
Enzymes perform the critical task of lowering a reaction's activation energy—that is, the amount of energy that must be put in for the reaction to begin. Enzymes work by binding to reactant molecules and holding them in such a way that the chemical bond-breaking and bond-forming processes take place more readily.
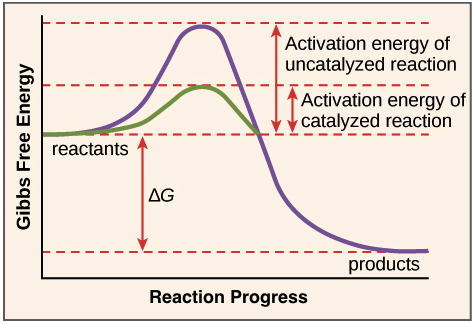
_Image modified from "Potential, kinetic, free, and activation energy: Figure 5," by OpenStax College, Biology, CC BY 3.0._
To clarify one important point, enzymes don’t change a reaction’s ∆G value. That is, they don’t change whether a reaction is energy-releasing or energy-absorbing overall. That's because enzymes don’t affect the free energy of the reactants or products.
Instead, enzymes lower the energy of the transition state, an unstable state that products must pass through in order to become reactants. The transition state is at the top of the energy "hill" in the diagram above.
Active sites and substrate specificity
To catalyze a reaction, an enzyme will grab on (bind) to one or more reactant molecules. These molecules are the enzyme's substrates.
In some reactions, one substrate is broken down into multiple products. In others, two substrates come together to create one larger molecule or to swap pieces. In fact, whatever type of biological reaction you can think of, there is probably an enzyme to speed it up!
The part of the enzyme where the substrate binds is called the active site (since that’s where the catalytic “action” happens).

Image modified from "Enzymes: Figure 2," by OpenStax College, Biology, CC BY 3.0.
Proteins are made of units called amino acids, and in enzymes that are proteins, the active site gets its properties from the amino acids it's built out of. These amino acids may have side chains that are large or small, acidic or basic, hydrophilic or hydrophobic.
The set of amino acids found in the active site, along with their positions in 3D space, give the active site a very specific size, shape, and chemical behavior. Thanks to these amino acids, an enzyme's active site is uniquely suited to bind to a particular target—the enzyme's substrate or substrates—and help them undergo a chemical reaction.
Environmental effects on enzyme function
Because active sites are finely tuned to help a chemical reaction happen, they can be very sensitive to changes in the enzyme’s environment. Factors that may affect the active site and enzyme function include:
Temperature. A higher temperature generally makes for higher rates of reaction, enzyme-catalyzed or otherwise. However, either increasing or decreasing the temperature outside of a tolerable range can affect chemical bonds in the active site, making them less well-suited to bind substrates. Very high temperatures (for animal enzymes, above \[40 \] \[ ^{\circ}\text C\] or \[104\] \[^{\circ}\text F\]) may cause an enzyme to denature, losing its shape and activity.\[^2\]
pH. pH can also affect enzyme function. Active site amino acid residues often have acidic or basic properties that are important for catalysis. Changes in pH can affect these residues and make it hard for substrates to bind. Enzymes work best within a certain pH range, and, as with temperature, extreme pH values (acidic or basic) can make enzymes denature.
Induced fit
The matching between an enzyme's active site and the substrate isn’t just like two puzzle pieces fitting together (though scientists once thought it was, in an old model called the “lock-and-key” model).
Instead, an enzyme changes shape slightly when it binds its substrate, resulting in an even tighter fit. This adjustment of the enzyme to snugly fit the substrate is called induced fit.
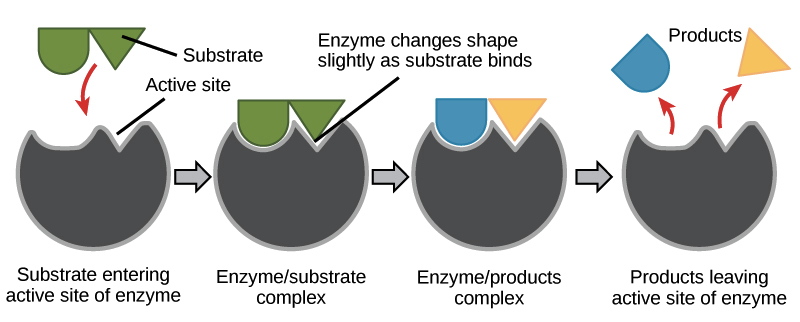
Image modified from "Enzymes: Figure 2," by OpenStax College, Biology, CC BY 3.0.
When an enzyme binds to its substrate, we know it lowers the activation energy of the reaction, allowing it to happen more quickly. But, you may wonder, what does the enzyme actually do to the substrate to make the activation energy lower?
The answer depends on the enzyme. Some enzymes speed up chemical reactions by bringing two substrates together in the right orientation. Others create an environment inside the active site that's favorable to the reaction (for instance, one that's slightly acidic or non-polar). The enzyme-substrate complex can also lower activation energy by bending substrate molecules in a way that facilitates bond-breaking, helping to reach the transition state.
Finally, some enzymes lower activation energies by taking part in the chemical reaction themselves. That is, active site residues may form temporary covalent bonds with substrate molecules as part of the reaction process.
An important word here is "temporary." In all cases, the enzyme will return to its original state at the end of the reaction—it won't stay bound to the reacting molecules. In fact, a hallmark property of enzymes is that they aren't altered by the reactions they catalyze. When an enzyme is done catalyzing a reaction, it just releases the product (or products) and is ready for the next cycle of catalysis.
Enzymes review
Key Terms
| Term | Meaning |
|---|---|
| Catalyst | A substance that speeds up a chemical reaction without being changed |
| Enzyme | A biological catalyst (usually a protein) |
| Substrate | The reactant molecule that an enzyme works on |
| Active site | The part of the enzyme where the substrate binds |
Enzyme structure and function
Enzymes are catalysts. They are usually proteins, though some RNA molecules act as enzymes too.
Enzymes lower the activation energy of a reaction - that is the required amount of energy needed for a reaction to occur. They do this by binding to a substrate and holding it in a way that allows the reaction to happen more efficiently.
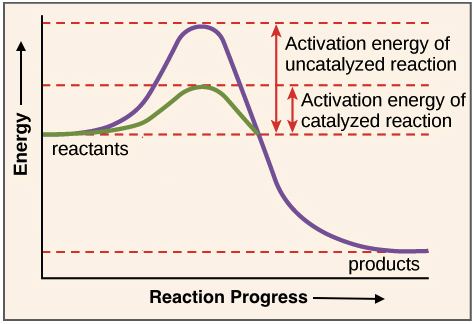
Image modified from OpenStax, CC BY 3.0.
The part of the enzyme where the substrate binds is called the active site. Here, the enzyme changes shape slightly, fitting tightly with the substrate and forming the enzyme/substrate complex.
Factors affecting enzyme activity
Enzyme activity can be affected by a variety of factors, such as temperature, pH, and concentration.
Enzymes work best within specific temperature and pH ranges, and sub-optimal conditions can cause an enzyme to lose its ability to bind to a substrate.
Temperature: Raising temperature generally speeds up a reaction, and lowering temperature slows down a reaction. However, extreme high temperatures can cause an enzyme to lose its shape (denature) and stop working.
pH: Each enzyme has an optimum pH range. Changing the pH outside of this range will slow enzyme activity. Extreme pH values can cause enzymes to denature.
Enzyme concentration: Increasing enzyme concentration will speed up the reaction, as long as there is substrate available to bind to. Once all of the substrate is bound, the reaction will no longer speed up, since there will be nothing for additional enzymes to bind to.
Substrate concentration: Increasing substrate concentration also increases the rate of reaction to a certain point. Once all of the enzymes have bound, any substrate increase will have no effect on the rate of reaction, as the available enzymes will be saturated and working at their maximum rate.
Common mistakes and misconceptions
- Enzymes are "specific." Each type of enzyme typically only reacts with one, or a couple, of substrates. Some enzymes are more specific than others and will only accept one particular substrate. Other enzymes can act on a range of molecules, as long as they contain the type of bond or chemical group that the enzyme targets.
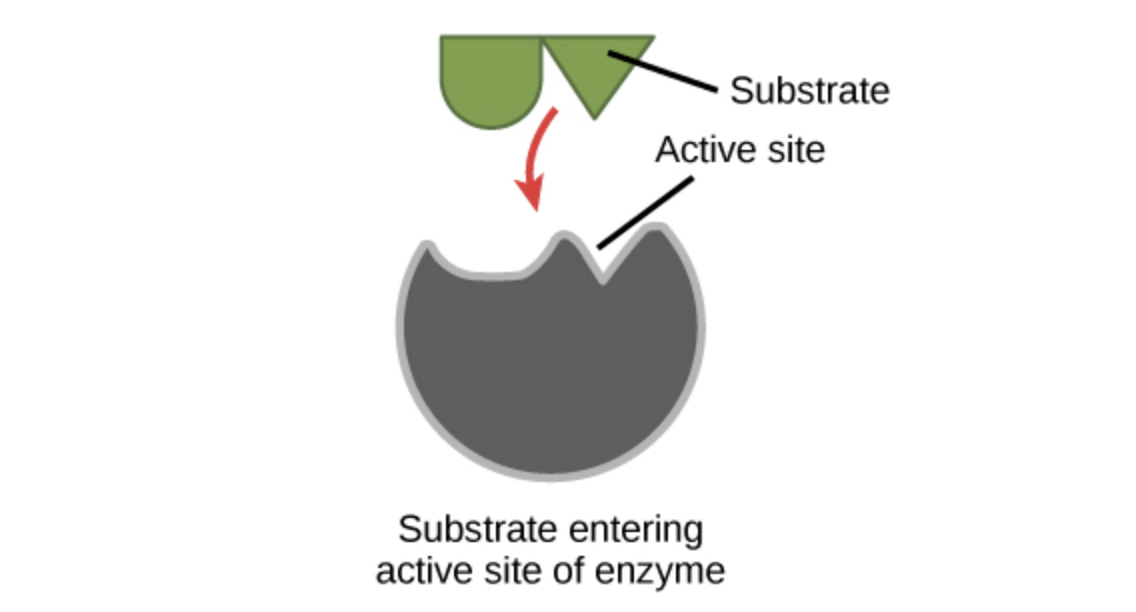
Image modified from "Enzymes: Figure 2," by OpenStax College, Biology, CC BY 3.0.
- Enzymes are reusable. Enzymes are not reactants and are not used up during the reaction. Once an enzyme binds to a substrate and catalyzes the reaction, the enzyme is released, unchanged, and can be used for another reaction. This means that for each reaction, there does not need to be a 1:1 ratio between enzyme and substrate molecules.
