Lactic acid fermentation
Alcohol or ethanol fermentation
Fermentation and anaerobic respiration
Introduction
Ever wonder how yeast ferment barley malt into beer? Or how your muscles keep working when you're exercising so hard that they're very low on oxygen?
Both of these processes can happen thanks to alternative glucose breakdown pathways that occur when normal, oxygen-using (aerobic) cellular respiration is not possible—that is, when oxygen isn't around to act as an acceptor at the end of the electron transport chain. These fermentation pathways consist of glycolysis with some extra reactions tacked on at the end. In yeast, the extra reactions make alcohol, while in your muscles, they make lactic acid.
Fermentation is a widespread pathway, but it is not the only way to get energy from fuels anaerobically (in the absence of oxygen). Some living systems instead use an inorganic molecule other than \[\text{O}_2\], such as sulfate, as a final electron acceptor for an electron transport chain. This process, called anaerobic cellular respiration, is performed by some bacteria and archaea.
In this article, we'll take a closer look at anaerobic cellular respiration and at the different types of fermentation.
Anaerobic cellular respiration
Anaerobic cellular respiration is similar to aerobic cellular respiration in that electrons extracted from a fuel molecule are passed through an electron transport chain, driving \[\text{ATP}\] synthesis. Some organisms use sulfate \[(\text{SO}_4^{2-})\] as the final electron acceptor at the end ot the transport chain, while others use nitrate \[(\text{NO}_{3}^-)\], sulfur, or one of a variety of other molecules\[^1\].
What kinds of organisms use anaerobic cellular respiration? Some prokaryotes—bacteria and archaea—that live in low-oxygen environments rely on anaerobic respiration to break down fuels. For example, some archaea called methanogens can use carbon dioxide as a terminal electron acceptor, producing methane as a by-product. Methanogens are found in soil and in the digestive systems of ruminants, a group of animals including cows and sheep.
Similarly, sulfate-reducing bacteria and Archaea use sulfate as a terminal electron acceptor, producing hydrogen sulfide \[(\text H_2\text S)\] as a byproduct. The image below is an aerial photograph of coastal waters, and the green patches indicate an overgrowth of sulfate-reducing bacteria.

Image credit: "Metabolism without oxygen: Figure 1," OpenStax College, Biology, CC BY 3.0; Modification of work by NASA/Jeff Schmaltz, MODIS Land Rapid Response Team at NASA GSFC, Visible Earth Catalog of NASA images.
Fermentation
Fermentation is another anaerobic (non-oxygen-requiring) pathway for breaking down glucose, one that's performed by many types of organisms and cells. In fermentation, the only energy extraction pathway is glycolysis, with one or two extra reactions tacked on at the end.
Fermentation and cellular respiration begin the same way, with glycolysis. In fermentation, however, the pyruvate made in glycolysis does not continue through oxidation and the citric acid cycle, and the electron transport chain does not run. Because the electron transport chain isn't functional, the \[\text{NADH}\] made in glycolysis cannot drop its electrons off there to turn back into \[\text{NAD}^+\]
The purpose of the extra reactions in fermentation, then, is to regenerate the electron carrier \[\text{NAD}^+\] from the \[\text{NADH}\] produced in glycolysis. The extra reactions accomplish this by letting \[\text{NADH}\] drop its electrons off with an organic molecule (such as pyruvate, the end product of glycolysis). This drop-off allows glycolysis to keep running by ensuring a steady supply of \[\text{NAD}^+\].
Lactic acid fermentation
In lactic acid fermentation, \[\text{NADH}\] transfers its electrons directly to pyruvate, generating lactate as a byproduct. Lactate, which is just the deprotonated form of lactic acid, gives the process its name. The bacteria that make yogurt carry out lactic acid fermentation, as do the red blood cells in your body, which don’t have mitochondria and thus can’t perform cellular respiration.
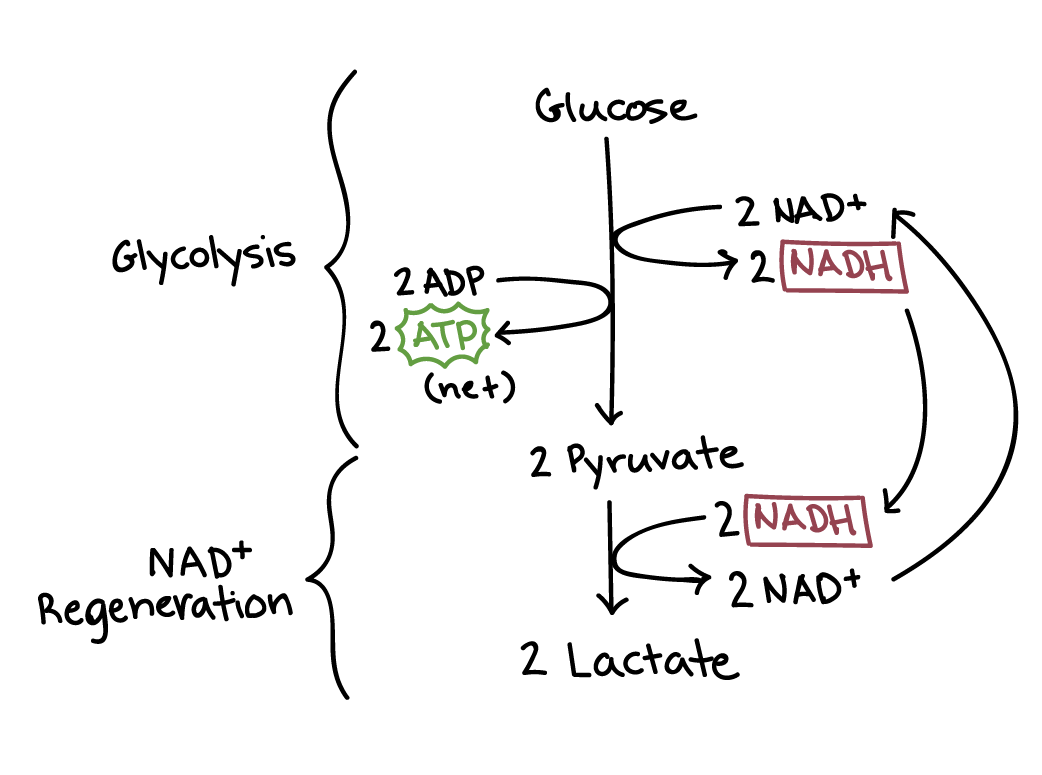
Muscle cells also carry out lactic acid fermentation, though only when they have too little oxygen for aerobic respiration to continue—for instance, when you’ve been exercising very hard. It was once thought that the accumulation of lactate in muscles was responsible for soreness caused by exercise, but recent research suggests this is probably not the case.
Lactic acid produced in muscle cells is transported through the bloodstream to the liver, where it’s converted back to pyruvate and processed normally in the remaining reactions of cellular respiration.
Alcohol fermentation
Another familiar fermentation process is alcohol fermentation, in which \[\text{NADH}\] donates its electrons to a derivative of pyruvate, producing ethanol.
Going from pyruvate to ethanol is a two-step process. In the first step, a carboxyl group is removed from pyruvate and released in as carbon dioxide, producing a two-carbon molecule called acetaldehyde. In the second step, \[\text{NADH}\] passes its electrons to acetaldehyde, regenerating \[\text{NAD}^+\] and forming ethanol.
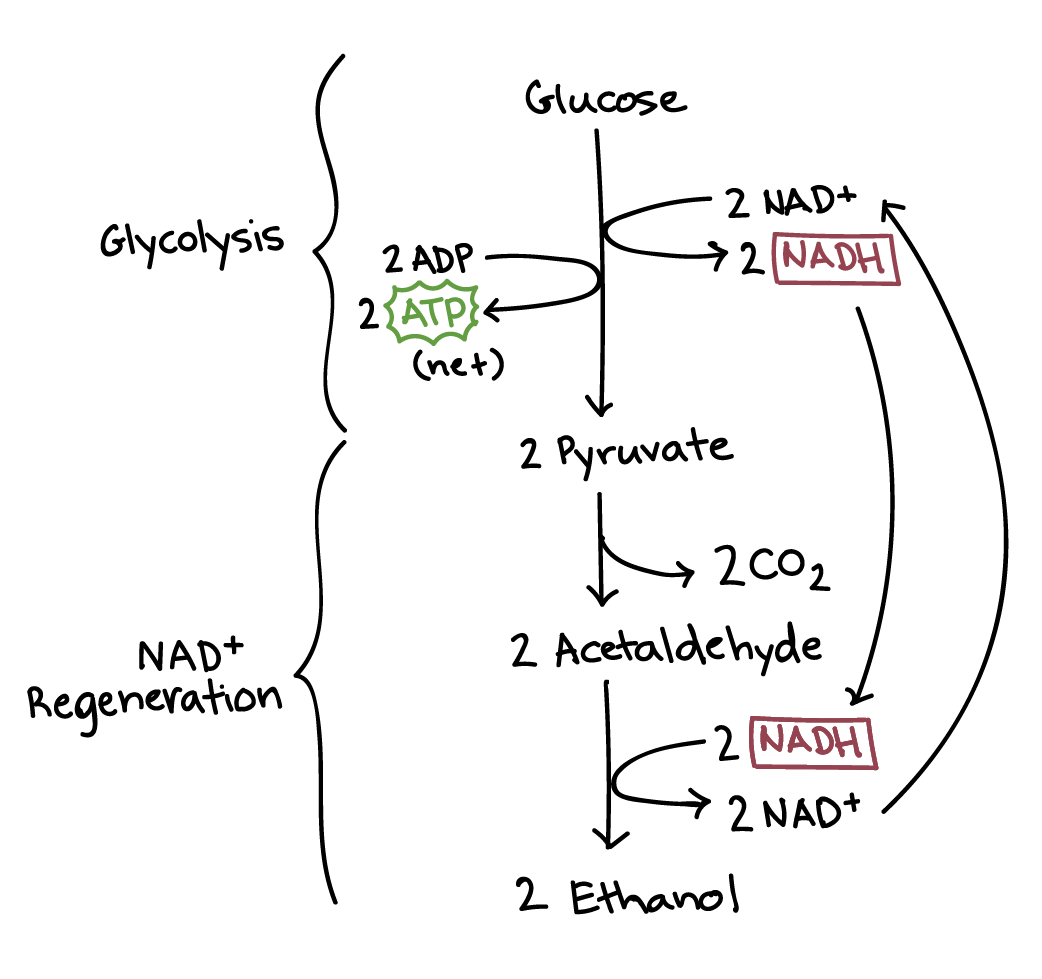
Alcohol fermentation by yeast produces the ethanol found in alcoholic drinks like beer and wine. However, alcohol is toxic to yeasts in large quantities (just as it is to humans), which puts an upper limit on the percentage alcohol in these drinks. Ethanol tolerance of yeast ranges from about \[5\] percent to \[21\] percent, depending on the yeast strain and environmental conditions.
Facultative and obligate anaerobes
Many bacteria and archaea are facultative anaerobes, meaning they can switch between aerobic respiration and anaerobic pathways (fermentation or anaerobic respiration) depending on the availability of oxygen. This approach allows lets them get more ATP out of their glucose molecules when oxygen is around—since aerobic cellular respiration makes more ATP than anaerobic pathways—but to keep metabolizing and stay alive when oxygen is scarce.
Other bacteria and archaea are obligate anaerobes, meaning they can live and grow only in the absence of oxygen. Oxygen is toxic to these microorganisms and injures or kills them on exposure. For instance, the Clostridium bacteria that are responsible for botulism (a form of food poisoning) are obligate anaerobes\[^2\]. Recently, some multicellular animals have even been discovered in deep-sea sediments that are free of oxygen\[^{3,4}\].
Connections between cellular respiration and other pathways
Introduction
So far, we’ve spent a lot of time describing the pathways used to break down glucose. When you sit down for lunch, you might have a turkey sandwich, a veggie burger, or a salad, but you’re probably not going to dig in to a bowl of pure glucose. How, then, are the other components of food – such as proteins, lipids, and non-glucose carbohydrates – broken down to generate ATP?
As it turns out, the cellular respiration pathways we’ve already seen are central to the extraction of energy from all these different molecules. Amino acids, lipids, and other carbohydrates can be converted to various intermediates of glycolysis and the citric acid cycle, allowing them to slip into the cellular respiration pathway through a multitude of side doors. Once these molecules enter the pathway, it makes no difference where they came from: they’ll simply go through the remaining steps, yielding NADH, FADH\[_2\], and ATP.
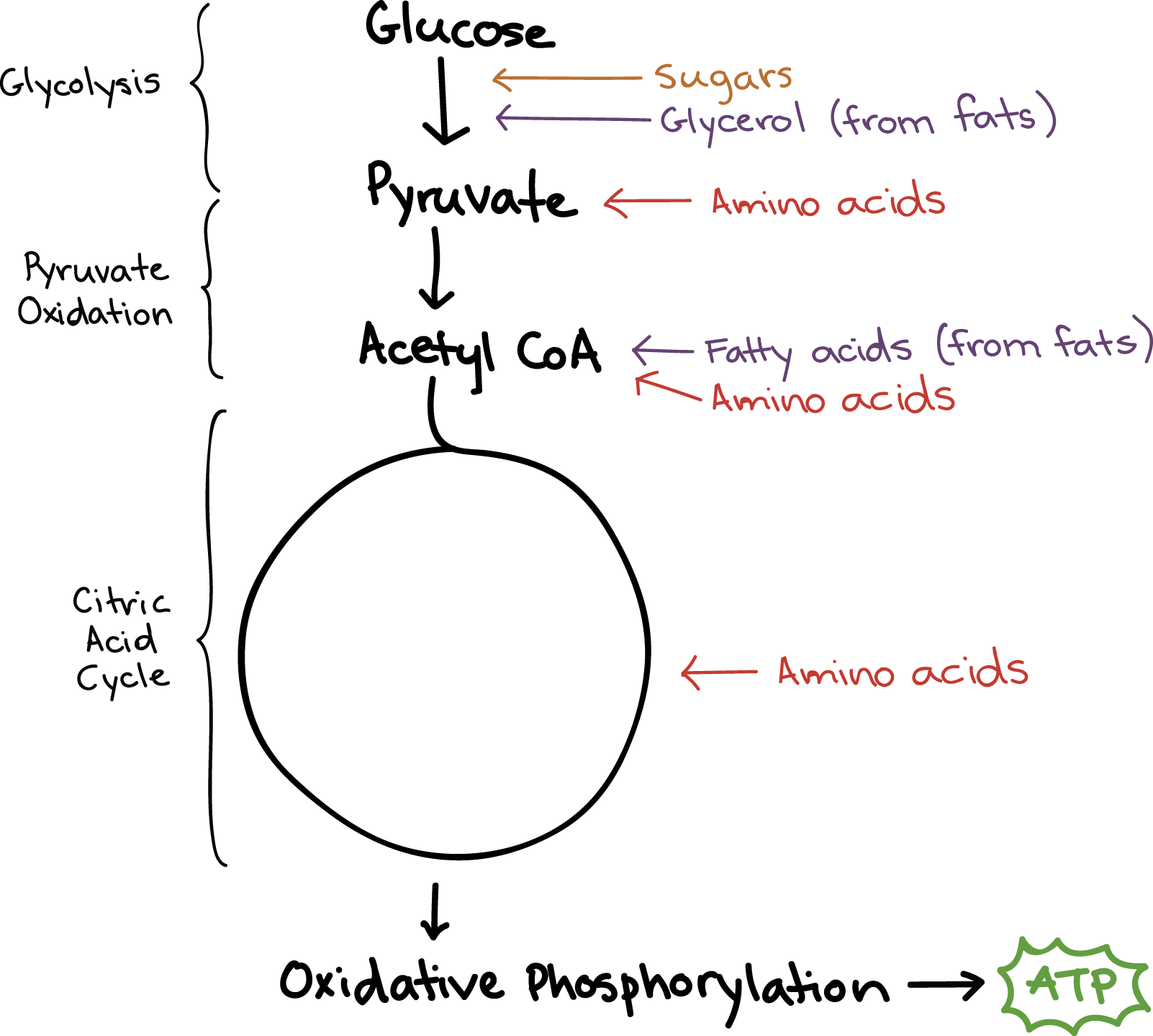
In addition, not every molecule that enters cellular respiration will complete the entire pathway. Just as various types of molecules can feed into cellular respiration through different intermediates, so intermediates of glycolysis and the citric acid cycle may be removed at various stages and used to make other molecules. For instance, many intermediates of glycolysis and the citric acid cycle are used in the pathways that build amino acids\[^1\].
In the sections below, we’ll look at a few examples of how different non-glucose molecules can enter cellular respiration.
How carbohydrates enter the pathway
Most carbohydrates enter cellular respiration during glycolysis. In some cases, entering the pathway simply involves breaking a glucose polymer down into individual glucose molecules. For instance, the glucose polymer glycogen is made and stored in both liver and muscle cells in our bodies. If blood sugar levels drop, the glycogen will be broken down into phosphate-bearing glucose molecules, which can easily enter glycolysis.
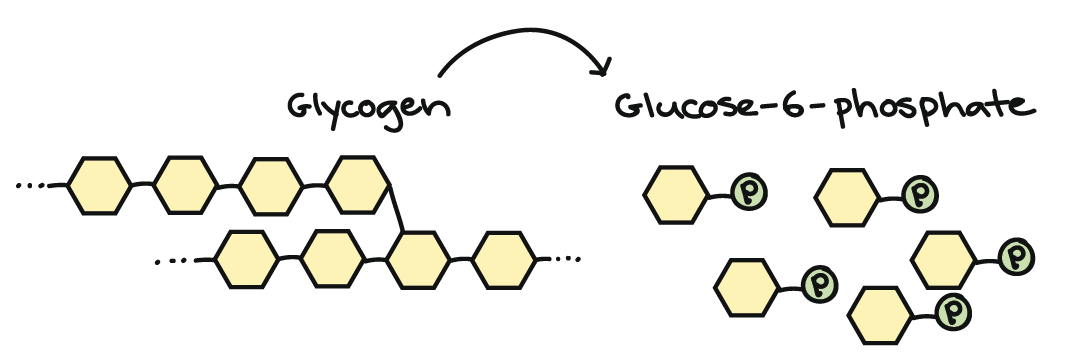
Non-glucose monosaccharides can also enter glycolysis. For instance, sucrose (table sugar) is made up of glucose and fructose. When this sugar is broken down, the fructose can easily enter glycolysis: addition of a phosphate group turns it into fructose-6-phosphate, the third molecule in the glycolysis pathway\[^2\]. Because it enters so close to the top of the pathway, fructose yields the same number of ATP as glucose during cellular respiration.
How proteins enter the pathway
When you eat proteins in food, your body has to break them down into amino acids before they can be used by your cells. Most of the time, amino acids are recycled and used to make new proteins, not oxidized for fuel.
However, if there are more amino acids than the body needs, or if cells are starving, some amino acids will get broken down for energy via cellular respiration. In order to enter cellular respiration, amino acids must first have their amino group removed. This step makes ammonia \[(\text{NH}_3)\] as a waste product, and in humans and other mammals, the ammonia is converted to urea and removed from the body in urine.
Once they’ve been deaminated, different amino acids enter the cellular respiration pathways at different stages. The chemical properties of each amino acid determine what intermediate it can be most easily converted into.
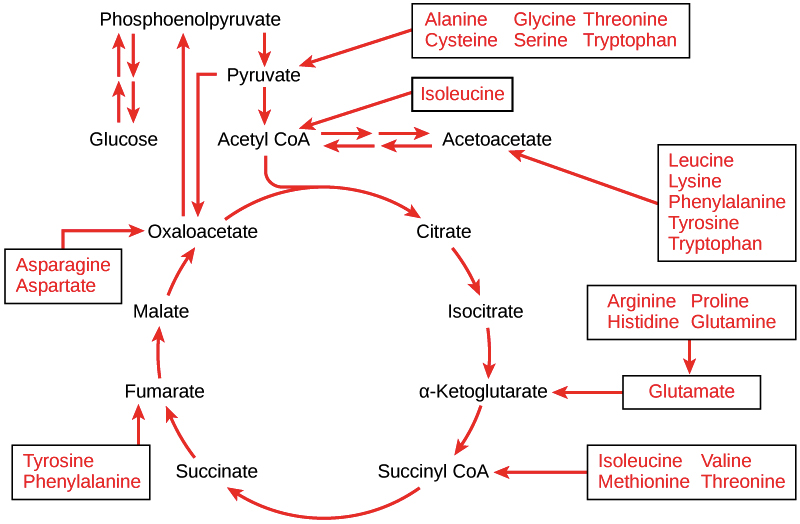
Image credit: "Connections of carbohydrate, protein, and lipid metabolic pathways," by OpenStax College, Biology, CC BY 4.0. Original work by Mikael Häggström
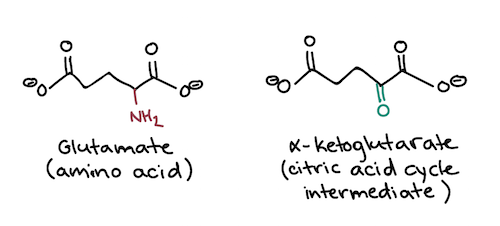
For example, the amino acid glutamate, which has a carboxylic acid side chain, gets converted into the citric acid cycle intermediate α-ketoglutarate. This point of entry for glutamate makes sense because both molecules have a similar structure with two carboxyl groups, as shown below\[^3\].
How lipids enter the pathway
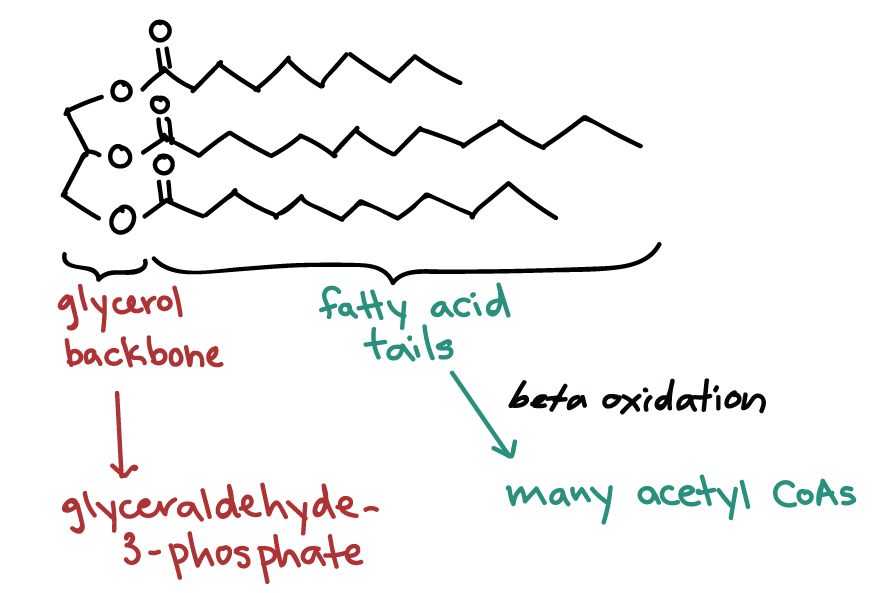
Fats, known more formally as triglycerides, can be broken down into two components that enter the cellular respiration pathways at different stages. A triglyceride is made up of a three-carbon molecule called glycerol, and of three fatty acid tails attached to the glycerol. Glycerol can be converted to glyceraldehyde-3-phosphate, an intermediate of glycolysis, and continue through the remainder of the cellular respiration breakdown pathway.
Fatty acids, on the other hand, must be broken down in a process called beta-oxidation, which takes place in the matrix of the mitochondria. In beta-oxidation, the fatty acid tails are broken down into a series of two-carbon units that combine with coenzyme A, forming acetyl CoA. This acetyl CoA feeds smoothly into the citric acid cycle.
Cellular respiration: It's a two-way street
We've thought a lot about how molecules can enter cellular respiration, but it's also important to consider how they can exit. Molecules in the cellular respiration pathway can be pulled out at many stages and used to build other molecules, including amino acids, nucleotides, lipids, and carbohydrates.
To give just one example, acetyl CoA (mentioned above) that's produced in cellular respiration can be diverted from the citric acid cycle and used to build the lipid cholesterol. Cholesterol forms the backbone of the steroid hormones in our bodies, such as testosterone and estrogens.
Whether it's better to "burn" molecules for fuel via cellular respiration or use them to build other molecules depends on the needs of the cell—and so does which specific molecules they're used to build!
Regulation of cellular respiration
Introduction
You can sometimes have too much of a good thing. For instance, consider ice cream sandwiches. Maybe you really like ice cream sandwiches and buy a bunch of them at the store. If you’re very hungry, that might be a good choice: you can eat them all quickly, before they melt. If you’re only a little hungry, though, that might be a bad choice: most of the sandwiches will melt uneaten, at which point you will have wasted some money.
Cells face a related problem when they break down fuels, such as glucose, to produce ATP. If the cell’s supply of ATP is low, it would do well to break down glucose as quickly as possible, replenishing the ATP it needs to “keep the lights on.” If the supply of ATP is high, on the other hand, it might not be such a good idea to oxidize glucose at top speed. ATP is an unstable molecule, and if it sits around in the cell too long, it’s likely to spontaneously hydrolyze back to ADP. This is like the case of the melted ice cream sandwich: the cell has spent glucose to make ATP, and that ATP ends up going to waste.
It’s important for a cell to carefully match the activity of its fuel breakdown (pathways to its energy needs at a given moment. Here, we'll see how cells turn cellular respiration pathways “up” or “down” in response to ATP levels and other metabolic signals.
Allosteric enzymes and pathway control
How is the activity of a pathway controlled? In many cases, pathways are regulated through enzymes that catalyze individual steps of the pathway. If the enzyme for a particular step is active, that step can take place quickly, but if the enzyme is inactive, the step will happen slowly or not at all. Thus, if a cell wants to control the activity of a metabolic pathway, it needs to regulate the activity of one or more of the enzymes in that pathway.
The primary target for regulation of a biochemical pathway is often the enzyme that catalyzes the pathway’s first committed step (that is, the first step that is not readily reversible). The concept of a committed step can get a little complicated when there are many intersecting metabolic pathways, as in cellular respiration, but this is still a useful idea to keep in mind.
How are the enzymes that control metabolic pathways regulated? A number cellular respiration enzymes are controlled by the binding of regulatory molecules at one or more allosteric sites. (An allosteric site is just a regulatory site other than the active site.) Binding of a regulator to the allosteric site of an enzyme changes its structure, making it more or less active.
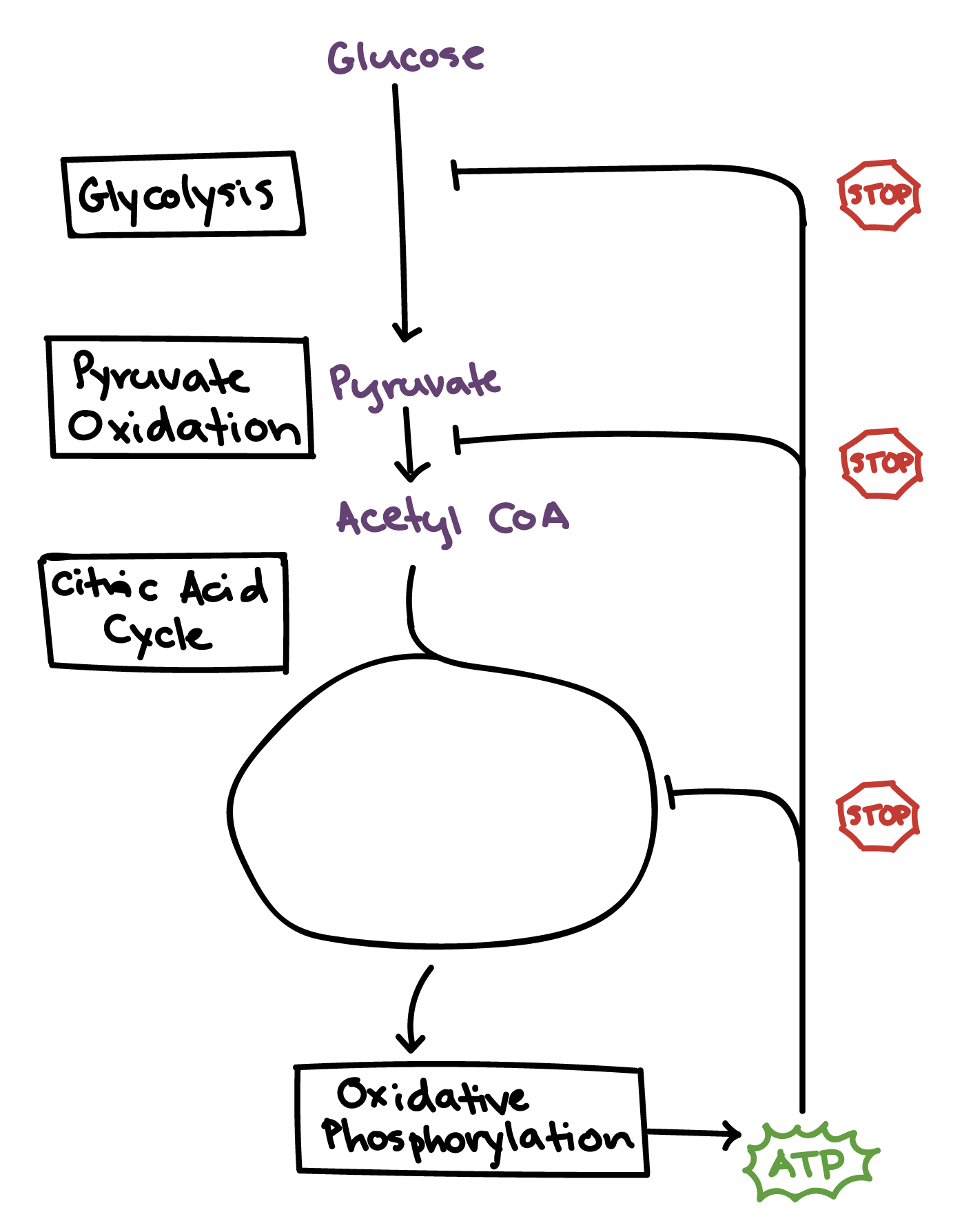
The molecules that bind cellular respiration enzymes act as signals, giving the enzyme information about the cell's energy state. ATP, ADP, and NADH are examples of molecules that regulate cellular respiration enzymes. ATP, for instance, is a "stop" signal: high levels mean that the cell has enough ATP and does not need to make more through cellular respiration. This is a case of feedback inhibition, in which a product "feeds back" to shut down its pathway.
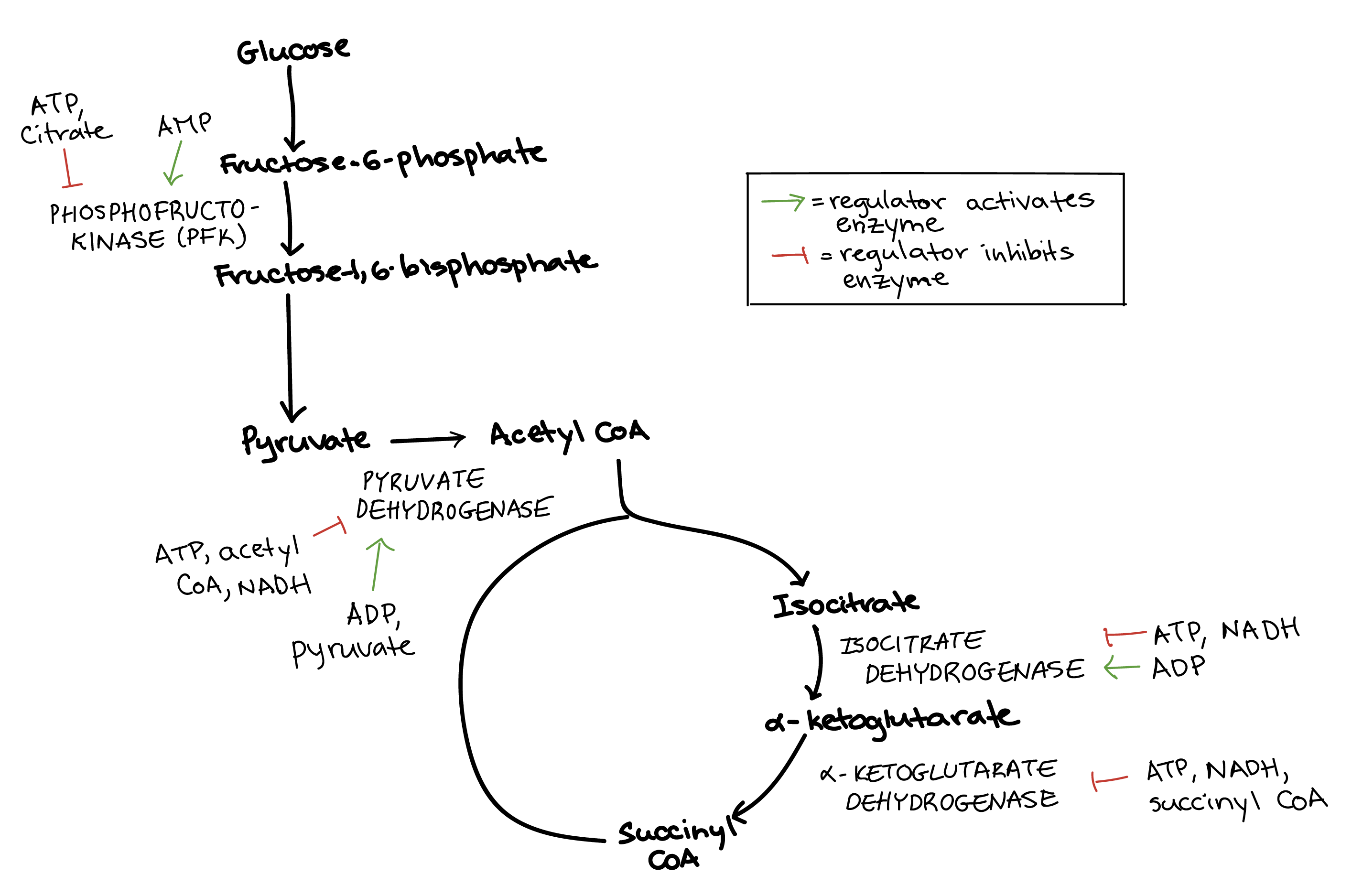
Regulation of glycolysis
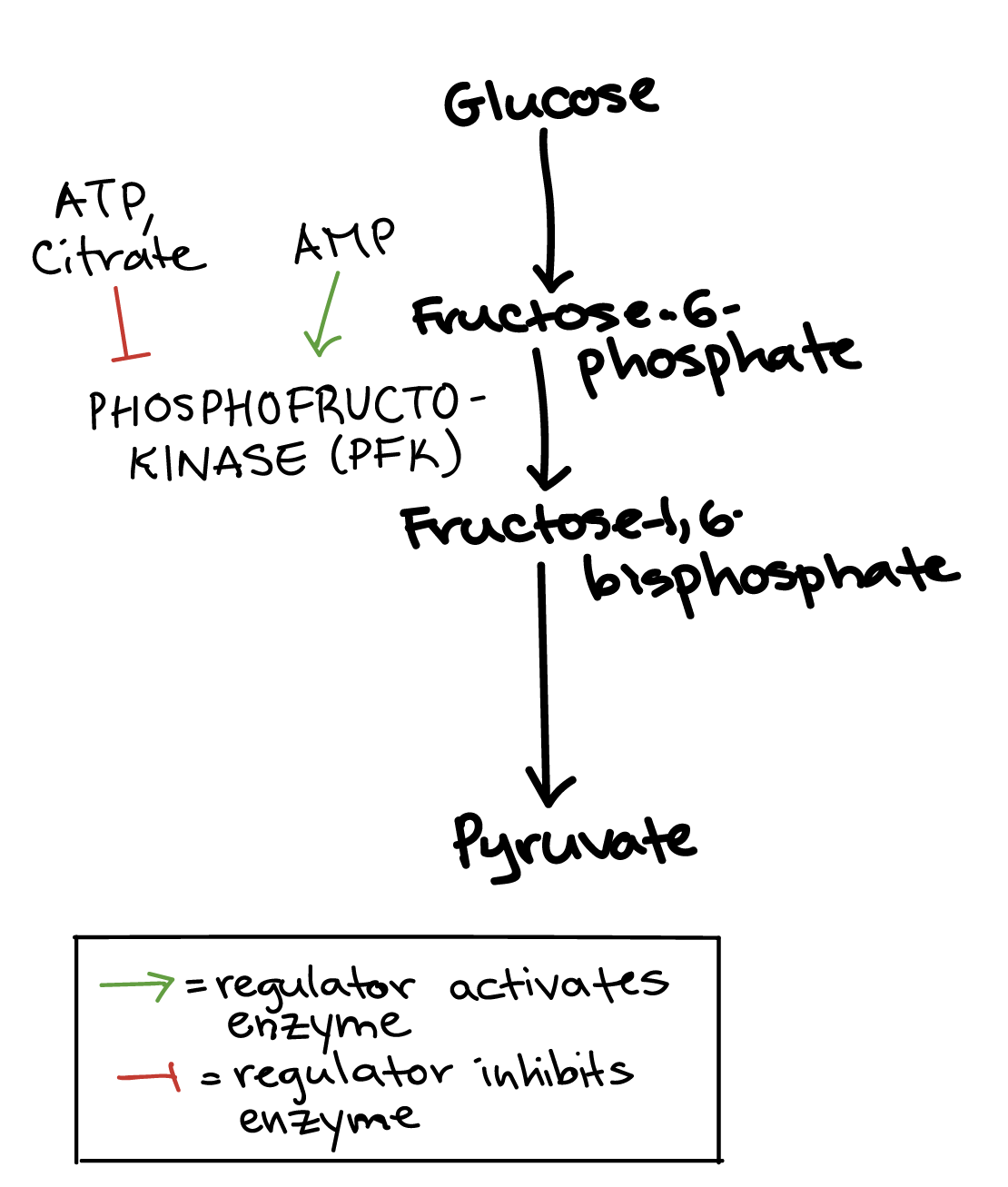
Several steps in glycolysis are regulated, but the most important control point is the third step of the pathway, which is catalyzed by an enzyme called phosphofructokinase (PFK). This reaction is the first committed step, making PFK a central target for regulation of the glycolysis pathway as a whole\[^1\].
PFK is regulated by ATP, an ADP derivative called AMP, and citrate, as well as some other molecules we won't discuss here.
ATP. ATP is a negative regulator of PFK, which makes sense: if there is already plenty of ATP in the cell, glycolysis does not need to make more.
AMP. Adenosine monophosphate (AMP) is a positive regulator of PFK. When a cell is very low on ATP, it will start squeezing more ATP out of ADP molecules by converting them to ATP and AMP (ADP + ADP \[\rightarrow\] ATP + AMP). High levels of AMP mean that the cell is starved for energy, and that glycolysis must run quickly to replenish ATP\[^2\].
Citrate. Citrate, the first product of the citric acid cycle, can also inhibit PFK. If citrate builds up, this is a sign that glycolysis can slow down, because the citric acid cycle is backed up and doesn’t need more fuel.
Pyruvate oxidation
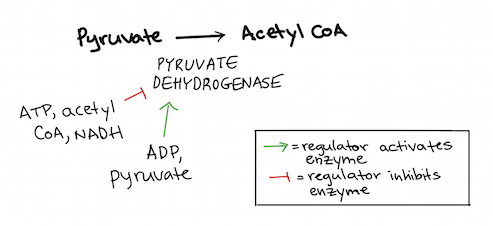
The next key control point comes after glycolysis, when pyruvate is converted to acetyl CoA. This conversion step is irreversible in many organisms and controls how much acetyl CoA “fuel” enters the citric acid cycle\[^3\]. The enzyme that catalyzes the conversion reaction is called pyruvate dehydrogenase.
ATP and NADH make this enzyme less active, while ADP makes it more active. So, more acetyl CoA is made when energy stores are low.
Pyruvate dehydrogenase is also activated by its substrate, pyruvate, and inhibited by its product, acetyl CoA. This ensures that acetyl CoA is made only when it’s needed (and when there's plenty of pyruvate available)\[^4\].
Citric acid cycle
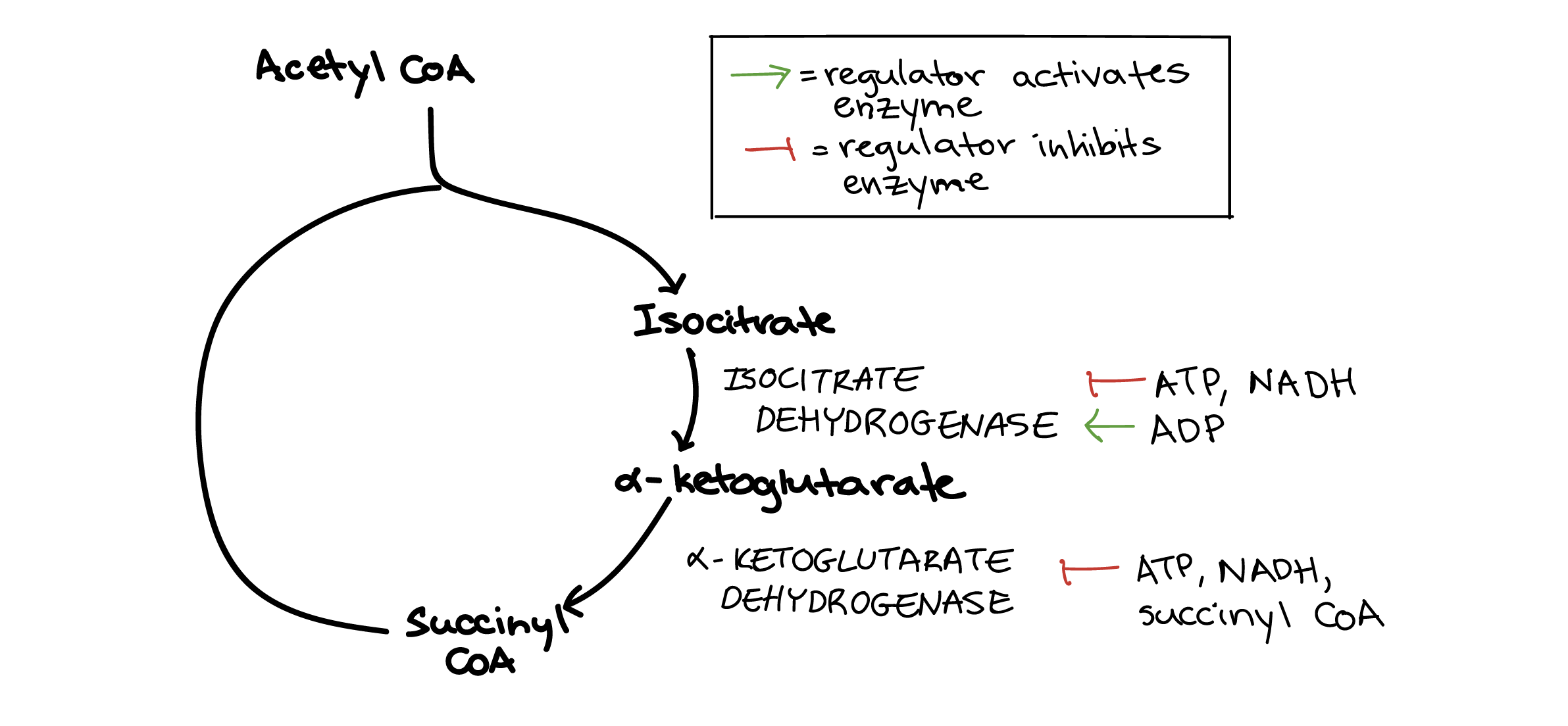
Entry into the citric acid cycle is largely controlled through pyruvate dehydrogenase (above), the enzyme that produces acetyl CoA. However, there are two additional steps in the cycle that are subject to regulation. These are the two steps in which carbon dioxide molecules are released, and also the steps at which the first two NADH molecules of the cycle are produced.
Isocitrate dehydrogenase controls the first of these two steps, turning a six-carbon molecule into a five-carbon molecule. This enzyme is inhibited by ATP and NADH, but activated by ADP.
α-Ketoglutarate dehydrogenase controls the second of these two steps, turning the five-carbon compound from the previous step into a four-carbon compound bound to CoA (succinyl CoA). This enzyme is inhibited by ATP, NADH, and several other molecules, including succinyl CoA itself.
Putting it all together
There are lots of other regulatory mechanisms for cellular respiration besides the ones we've discussed here. For instance, the speed of the electron transport chain is regulated by levels of ADP and ATP, and many other enzymes are subject to regulation. However, these examples give you a feel for the kind of logic and strategies cells use to regulate metabolic processes.
At each stage, we can see similar elements. For instance, we see feedback inhibition at many stages, at the level of pathways and of individual reactions. Monitoring of the cell's energy state through levels of molecules like ATP, ADP, AMP, and NADH is another common feature.
The diagram below summarizes the key enzymes we’ve discussed, along with some of their most important regulators.